| 1. Research Objectives | <<< Key Questions >>> | | How is the cell fate determined and how do the cells recognize their positions? | | How does this information translate into the development of structures? | | How do the cell wall properties change during the developmental processes and in response with external stimuli? | | How are the changes in cell wall properties perceived and transduced? |
 | | One of the key features of multicellular organisms is the coordination of different cell types in a spatiotemporal arrangement. How different cell types arise at appropriate places and at appropriate times are one of the most intensively investigated questions in modern developmental biology. However, the biology of stem cells and their inherent features both in plants and animals still remain enigmatic. Unlike animal cells, plant cells have a rigid cellulosic extracellular matrix, the cell walls providing physical support and communication routes with adjacent cells, which enables unique mechanisms for generating asymmetries and shaping tissues in plants. Furthermore, it is important to note that cell differentiation in plants does not correspond to terminal restriction in potency. Differentiated plant cells can change their fate quite easily if moved to a new position or placed in isolation, thus, providing a unique opportunity to investigate developmental plasticity and reprogramming. The long-term goal of our research is to investigate the molecular mechanisms of cell specification resulting in specific cellular architecture and understand dynamic changes in cell wall structure representing cellular activity in response with environmental stimuli. | |
| 2. Details and Directions of Research | | Aim 1. To identify spatiotemporal regulatory mechanisms and signaling networks that specify cellular identity and architecture. | 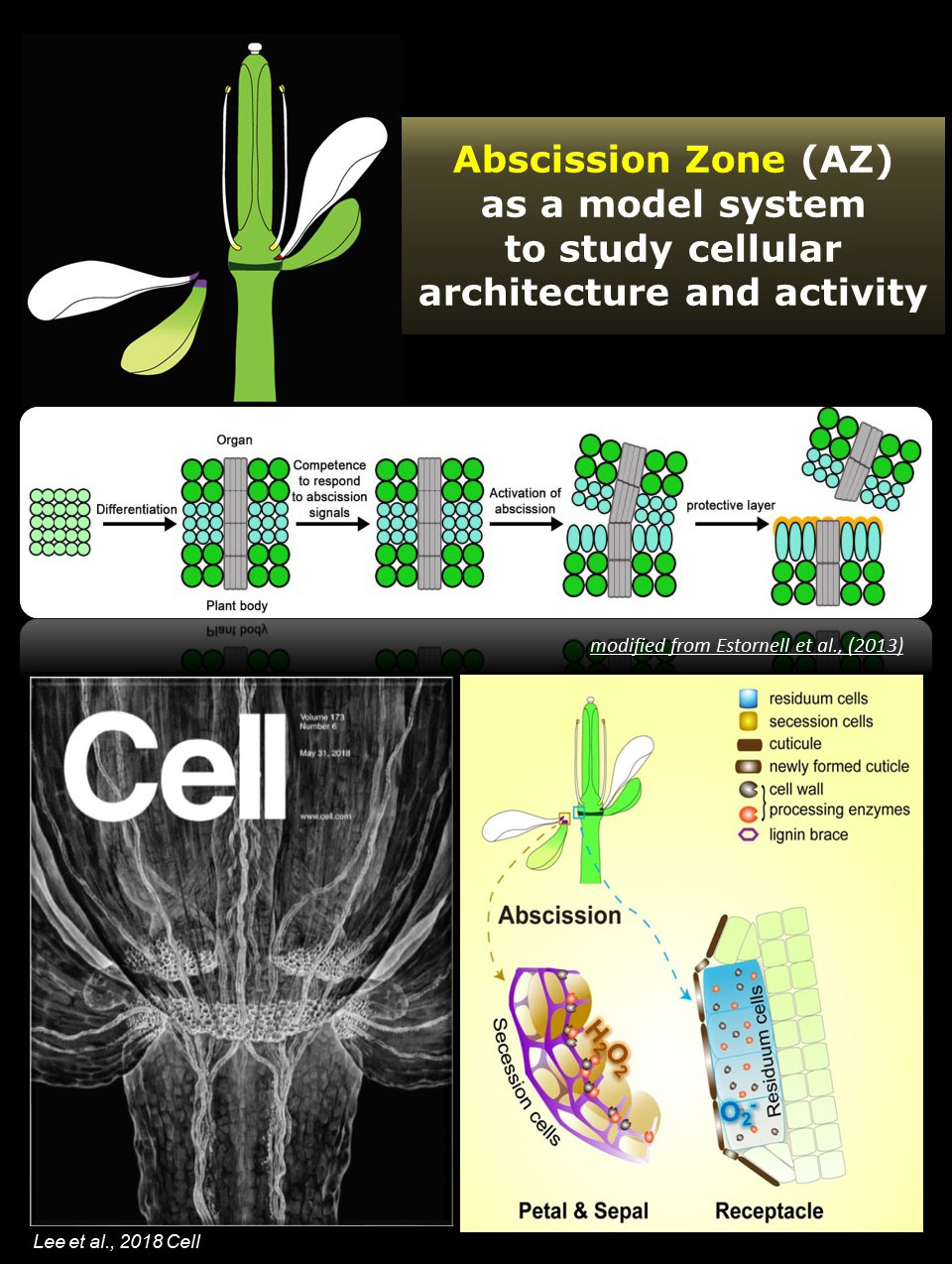 | | Our research aims at identifying the molecular mechanisms of cell specification and cell wall remodeling using the abscission zone (AZ) as a model system. I have recently shown that the AZ consists of two types of neighboring cells: residuum cells (RECs) and secession cells (SECs), which have spatiotemporally differential cellular functions and architecture to control cell wall processing, thereby ensuring precise abscission to discard organs (Lee et al., 2018). Considering unique features of AZ such as distinguished features of stage transition, substantial implications of external cues on the activation, and dramatic changes of cell wall architecture, further investigation of the molecular mechanism underlying AZ development will provide insight into the establishment and maintenance of cellular specificity, regulation of cellular state transition, and integration of environmental signals into the development program. Furthermore, the abscission process may be the only time when epidermal cell identity is specified de novo after embryogenesis. Research on the transdifferentiation events occurring in a single layer of RECs will allow circumventing the limitations imposed by genetic lethality and provide insights into the de novo specification of cellular identity, the effect of positional cues on cell fate, communication mechanisms between neighboring cells, and environmental influence on the formation of a new epidermis. | | | Aim 2. To investigate ROS-mediated signaling networks that regulate cell-type specificity and cell wall remodeling. |  | | I have shown that the AZ displays a cell-type-specific ROS distribution characterized by the accumulation of hydrogen peroxide in SECs and that of superoxide accumulation in RECs, which potentially plays a role in regulating cell state decisions (Lee et al., 2018). I identified that this cell-type specificity of ROS distribution was impaired in nevershed mutants showing non-specific ROS accumulation in both SECs and RECs. Interestingly, nevershed mutants also showed abnormal cellular activities with disturbed lignin formation in SECs, unexpected lignin deposition in RECs, and suppression of protective layer formation in RECs. These results suggest that the cell-type-specific ROS accumulation plays a critical role in the coordination of cellular activities in the AZ. To further understand the molecular mechanisms by which ROS signaling mediates cellular specificity, we will identify molecular players responsible for the cell-type-specific ROS distribution in the AZ and investigate the spatial control mechanisms for machinery involved in ROS homeostasis. | | | Aim 3. To investigate regulatory mechanisms for the localization, activity, and turnover of the cell wall proteins. |  | | The organization of the cell wall is regulated by secreted proteins as well as proteins localized at the plasma membrane. Whereas the regulatory mechanisms of membrane proteins have been adequately addressed, little is known about the mechanisms regulating the localization and activities of secreted proteins. I previously showed that localized activity of peroxidases is important in confining lignification and that these localizing factors are brought together by the CASPs (Lee et al., 2013). The Arabidopsis genome encodes for 5 CASPs and 34 CASPLs, all of which are expressed in a highly tissue-specific manner (Roppolo et al., 2014). Whether the CASPLs act as modulating factors to coordinate cell wall modifying enzymes in diverse cell types will be investigated. | | | Aim 4. To investigate cell wall dynamics and their roles in plant-microbe and plant-environmental interactions. | | This research will be further expanded to investigate cell wall dynamics and their roles in plant-microbe and plant-environmental interactions, which will be started with the comparative analysis of protective mechanisms during programmed abscission, wound healing, and immune response. Adventitious abscission can take place in response to external stimuli forming an AZ at a position that is not predetermined by the morphology of the plant. One of the interesting kind of adventitious abscission results from infections caused by the “shot-hole” diseases. The name “shot hole” is derived from the phenotype of the infected plant; the center of the infected site dries and falls out, leaving behind holes in the leaf that resemble the damage caused by gunshots. In shot hole disease, an abscission layer is formed around the infected tissue. What will happen if plants lose the enzymatic activity for separation and retain the ability of protective layer formation? In such a situation, the abscission layer would be a boundary between life and death, which could share molecular machinery with them in the boundary formation in response with wound and pathogen attack. This research will investigate similarities and differences in the protective boundary formation among programmed abscission, wound healing, and immune response by comparatively analyzing cell biological, biochemical, and genetic features. AZ-specific reporter systems will be used to identify genes that are commonly up-regulated at the boundary of wound and infection sites. Forward genetic screen based on the reporter system will be used to identify novel players that are involved in the defense-related boundary formation. Together with immunohistochemistry and functional analysis of mutants, this research will expand our knowledge of defense systems in plants and provide a comprehensive view of protective mechanisms. | | |