ISG15 is one of the most strongly induced genes after interferon treatment. It is also significantly induced by influenza B virus, lipopolysaccharide, and genotoxic stress. ISG15 is a 15-kDa ubiquitin (Ub)-like protein (Ubl) consisting of two Ub-related domains, whose amino acid sequences are about 30 % (N-terminal domain) and 36 % (C-terminal domain) identical to that of Ub, accounting for its cross-reactivity with affinity-purified anti-Ub antibodies. Several reports demonstrate that ISG15 is released by various cell types and can act as a cytokine leading to proliferation of NK cells. It has also been demonstrated that ISG15 is induced in the uterine endometrium during early pregnancy and is suggested to play a significant role in embryo implantation.
ISG15 is absent in yeast, nematode (Caenorhabditis), plant (Arabidopsis), and insect (Drosophila), indicating that the ISG15 conjugation system is restricted to higher animals with evolved IFN signaling. Most remarkably, the known data regarding the ISG15 system indicate that ISG15 modification (ISGylation) of proteins occurs in a manner analogous to ubiquitination or other Ubl modifications.
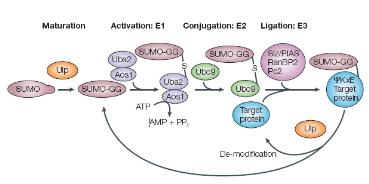
In human and rodent cells, ISG15 is expressed as precursor proteins with C-terminal amino acid extensions, which are processed prior to conjugation to expose the C-terminal Gly residue. UBE1L acts as ISG15-specific E1 activating enzyme, and UBC8 functions as an E2 conjugating enzyme for ISG15. An ISG15-specific ligase E3 has yet not been identified, but the IFN-induced Ub E3-like enzymes Rsp5 and CEB/Hc5 may function in ISG15 conjugation. Recently, a number of target proteins for ISGylation have been identified, which include PLCg1, ERK1, JAK1, STAT1, Serpin2A, and Ubc13. Unlike ubiquitin modification, however, little is known about the physiological consequences of ISG15 conjugation to target proteins.