[리뷰] Pectin Drives Cell Wall Morphogenesis Without Turgor Pressure
Yuree Leel 2020-07-08l 조회수 735
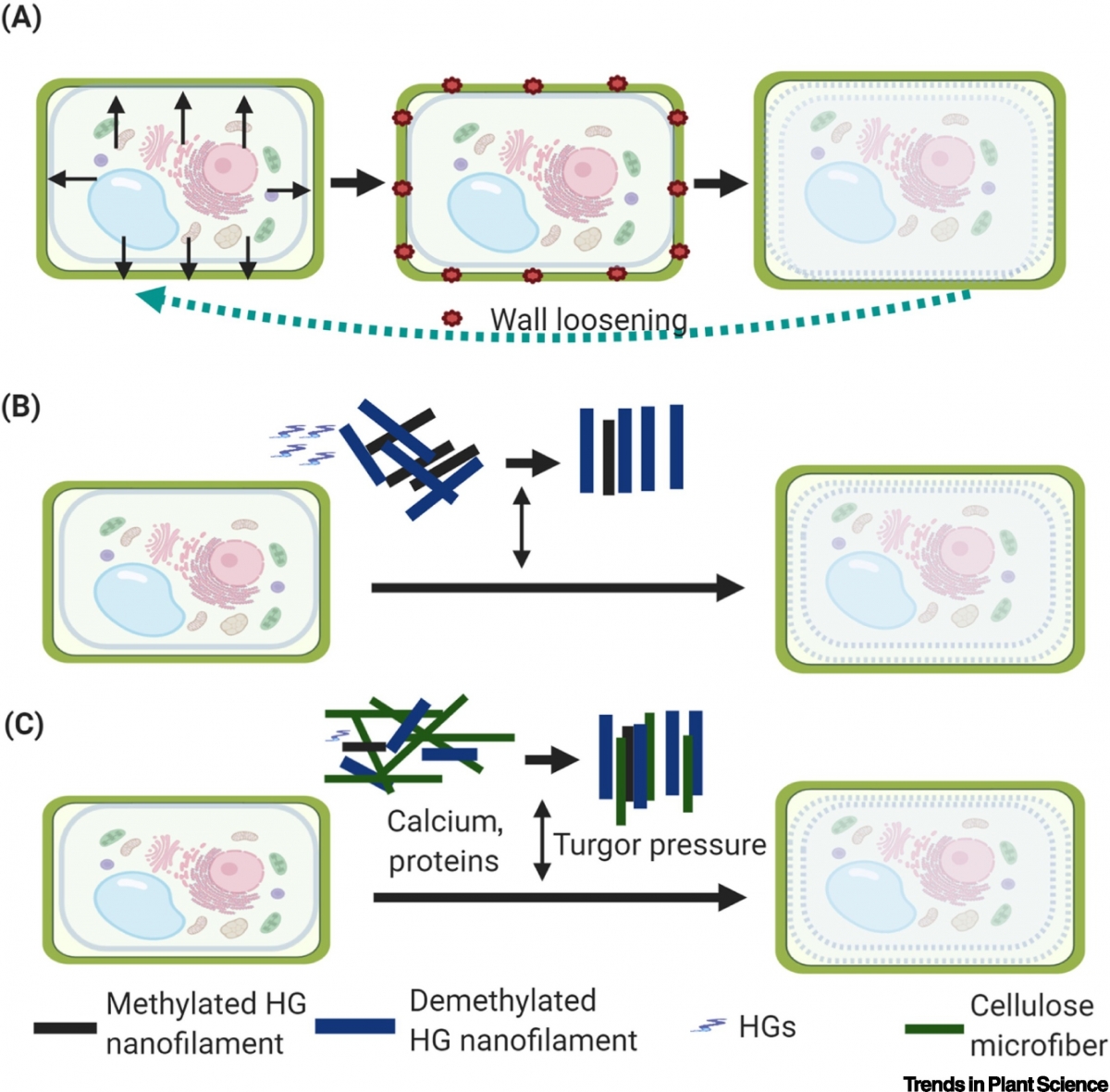
[Trends Plant Sci 2020 Jun;S1360-1385(20)30189-8]
Models of Forces Governing Plant Cell Wall Expansion.
(A) The traditional model in which turgor pressure drives cell wall expansion and morphogenesis. When turgor pressure is generated inside the cells, it causes cell wall loosening and expansion, and returns the cell wall to its original state when the turgor pressure is released. (B) The model proposed by Haas and colleagues in which pectin homogalacturonan (HG) methylation/demethylation and nanofilament remodeling provide the force that drives cell wall expansion. Pectin HG forms a quaternary nanofilament structure in the cell wall, and its methylation/demethylation determines the quaternary structure. Methylated HG is packed in hexagonal lattices whereas demethylated HG is packed in rectangular lattices. HG demethylation thus drives HG nanofilament expansion and cell wall growth. (C) The model of cell wall expansion proposed in this paper. Cell wall molecules interact with each other during the remodeling of cellulose microfibers and pectin HG nanofilaments which drives cell wall expansion and cell growth. Other molecules, including soluble proteins and calcium, may serve as signaling molecules in response to turgor pressure and internal/external stresses. The pectin–cellulose network plus turgor pressure together drive cell wall growth and control cell wall shape.
Abstract
How the plant cell wall expands and forms shapes is a long-standing mystery. Traditional thought is that turgor pressure drives these processes. However, a recent study by Haas and colleagues shows for the first time that the expansion of pectin homogalacturonan nanofilaments drives morphogenesis without turgor pressure in plant epidermal cells.
Concluding Remarks and Future Perspectives
This is the first observation that pectin forms nanofilament structures in intact cells. Based on the observation and their FEM model, Haas et al. demonstrate that HG methylation and demethylation result in switching of quaternary structures of HG nanofilaments, from a hexagonal to a rectangular lattice in the cell wall, leading to HG filament expansion, and that this is the primary force that shapes the cell wall and cell growth (Figure 1B). However, there are still many questions to be answered to fully understand the mechanisms driving cell wall biosynthesis and morphogenesis. Although HG nanofilament expansion provides power for cell wall expansion, it may not be the only driving force. Other forces such as traditionally hypothesized turgor pressure, as well as cellulose microfiber remodeling, may operate together with HG remodeling to control cell wall expansion and cell shape (Figure 1C). Observing the HG nanofilament quaternary is important, and finer (e.g., crystalline) structures will need studies to confirm the natural structure of each component and their interactions in the cell wall. In addition, although methylation/demethylation of pectin HG is a key driver of pectin filament remodeling and function, the molecular mechanism driving the switch between these two forms is unclear.